Project groups
Dr. Luigi Marongiu’s research focuses on understanding how nutrients can influence microbiome changes, which can lead to various health issues, including diabetes and cancer. In particular, he seeks to determine whether non-nutritive sweeteners (NNS), which are widespread in our food, can alter the phageome. Since phages can significantly influence the bacterial growth, they are a crucial element of the microbiome. Luigi Marongiu showed that NNS can compete with natural sugar in binding pockets of phage proteins involved in phage infection (Figure 1).
Figure 1: Docking analysis of the baseplate protein gp31 of Klebsiella phage 32. The natural ligand of this protein is maltose, but the non-nutritive sweeteners sucralose and steviol can also fit into the binding pocket, overlapping with maltose.
Phages exposed to NNS had an altered infectivity behavior compared to controls. Nutrients can significantly influence the delicate balance of our phageome, triggering a ripple effect throughout our bacteriome, immune system, and overall physiology (Figure 2). It’s a fascinating interplay that highlights how interconnected human health is!
Figure 2: The complex network of nutrition and microbiome. Non-nutritive sweeteners can (i.) interfere with phage infection and (ii.) boost genetic exchange (horizontal gene transfer), promoting the formation of pathovars (commensal bacteria carrying virulence genes). Non-nutritive sweeteners might also (iii.) stabilize phages that infect commensal species and (iv.) induce commensal bacteria carrying integrated phages (prophage induction). The combined effect is an increment in the prevalence of harmful bacteria in the intestinal tract, impairment of the immune response (which usually reduces the spread of harmful bacteria), and the promotion of altered cells (cancer precursors).
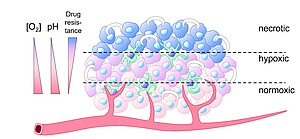
The research of Dr. Markus Burkard focuses on establishing animal-free gastrointestinal organoid models (malignant and non-malignant) as nutrition-related and oncological test systems. Organoids are generally a very powerful tool in preclinical research, individualized tumor therapy, and for the protection of animal life. To further increase the informative value of these models, (i.) realistic oxygen conditions are applied, for example to simulate the oxygen gradient in rapidly growing tumors (Figure 1), and (ii.) artificial extracellular matrices derived from animals are avoided.
Figure 1: Solid tumors usually consist of zones with heterogeneous oxygen supply. (i.) Relatively well-supplied rapidly proliferating peripheral areas, (ii.) hypoxic zones and niches with slowly proliferating but extremely therapy-resistant tumor cell clones and cancer stem cells, and (iii.) necrotic areas. The oxygen content decreases from the outside to the inside and runs parallel to a pH decrease and antiparallel to drug resistance. Green cells indicate infiltrating (mostly immunosuppressive) immune cells and white crescents indicate extracellular matrix surrounding the tumor.
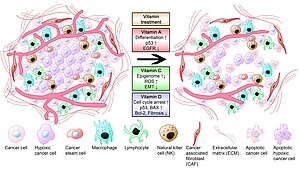
Organoid cultivation under realistic conditions enables the evaluation of promising treatment approaches such aspharmacological ascorbate and also of micronutrient effects on cancer cell viability and extracellular matrix formation (Figure 2)
Figure 2: Suggested effects of vitamins A, C, and D in pancreatic cancer therapy. Conventional therapies are still not very effective in pancreatic cancer particularly due to a dense tumor stroma that prevents sufficient drug delivery into the tumor. Vitamin A, C, and D reduce proangiogenic signaling. A combination of conventional therapies with various vitamins hypothetically improves therapeutic outcome. Vitamin A causes increased cancer cell differentiation, re-expression of p53, and a down-regulation of epidermal growth factor receptor (EGFR). Pharmacological vitamin C induces reactive oxygen species (ROS) formation, epigenome regulation, and inhibits epithelial mesenchymal transition. Vitamin D and its analogs reduce stromal fibrosis, induce cell cycle arrest, and apoptosis (see Piotrowsky et al. 2024). BAX: Bcl-2-like protein 4; Bcl-2: B-cell lymphoma 2.